Eye Drops Solution
Travoprost and Timolol maleate
Consumer Medicine Information (CMI) summary
The
full CMI on the next page has more details. If you are worried about using this medicine,
speak to your doctor or pharmacist.
1. Why am I using DuoTrav?
DuoTrav contains the active ingredients Travoprost and Timolol maleate. It is used
to treat certain types of eye conditions such as high pressure inside your eye and
open angle glaucoma (an eye condition caused by fluid buildup that damages the optic
nerve).
2. What should I know before I use DuoTrav?
Do not use if you have ever had an allergic reaction to DuoTrav or any of the ingredients
listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines,
or are pregnant or plan to become pregnant or are breastfeeding.
3. What if I am taking other medicines?
Some medicines may interfere with DuoTrav and affect how it works.
4. How do I use DuoTrav?
Put one drop of DuoTrav once daily into the affected eye(s).
Follow the instructions provided and use DuoTrav until your doctor tells you to stop.
5. What should I know while using DuoTrav?
|
Things you should do
|
Remind any doctor, dentist, pharmacist or specialist you visit that you are using
DuoTrav.
Remove your soft contact lenses before putting in DuoTrav.
Tell your doctor immediately if you notice any signs of an allergic reaction such
as skin rash, redness of skin or fever.
|
|
Things you should not do
|
Do not stop using this medicine suddenly.
Do not use DuoTrav in children.
Do not touch the dropper tip with your fingers or to your eye or any other surface.
|
|
Driving or using machines
|
DuoTrav may cause temporary blurred vision in some people.
Be careful before you drive or use any machines or tools until you know how DuoTrav
affects you.
|
|
Looking after your medicine
|
Store below 25 °C.
Keep it where young children cannot reach it.
|
6. Are there any side effects?
Common side effects include redness of the eye(s), eye pain, reduced vision, dry eye,
eye irritation, eye swelling, excessive secretion of tears from the eye(s).
Eye Drops Solution
Active ingredients:
Travoprost and Timolol maleate
Full Consumer Medicine Information (CMI)
This leaflet provides important information about using DuoTrav. You should also speak to your doctor or pharmacist if you would like further information
or if you have any concerns or questions about using DuoTrav.
Where to find information in this leaflet:
1. Why am I using DuoTrav?
DuoTrav contains the active ingredients
Travoprost
and
Timolol maleate
. Travoprost belongs to class of drugs called Prostaglandin analogs. Timolol maleate
belongs to class of drugs called beta-blockers.
DuoTrav is used to treat
certain types of eye conditions such as high pressure inside your eye and open angle
glaucoma (an eye condition caused by fluid buildup that damages the optic nerve).
DuoTrav helps to lower the increased eye pressure in two ways. The active ingredient
Travoprost helps in increasing the amount of fluid that drains from the eye and Timolol
maleate helps to decrease the production of fluid.
2. What should I know before I use DuoTrav?
Warnings
Do not use DuoTrav if:
you are allergic to Travoprost and Timolol maleate, or any of the ingredients listed
at the end of this leaflet. Always check the ingredients to make sure you can use
this medicine. Symptoms of allergic reaction may include shortness of breath, difficulty
breathing, swelling of the face, lips, tongue or other parts of the body, rash, hives.
you are pregnant or planning to become pregnant.
you suffer from any respiratory problems such as asthma (disease of airways) or a
history of asthma, or severe chronic obstructive pulmonary disease (serious lung condition
caused due to damage to the lungs)
you have any heart problems such as problems with your heart beat, problems with heart
function, any history of heart attack, low blood pressure or any other heart disease.
Check with your doctor if you:
have any problems with your blood vessels such as severe forms of Raynaud's disease
(a condition characterized by skin colour changes, affected part feels cold or numb,
skin ulcers).
use any other beta-blocker eye drop and/or another prostaglandin eye drop.
have diabetes or problems with low blood sugar levels or problems with your thyroid
gland.
suffer from myasthenia gravis (a serious disease that causes muscle weakness along
with symptoms such as dropping eyelid, double vision, difficulty in swallowing).
have a history of allergic problems, e.g. hives or hay fever.
suffer from any swelling of the eye or any diseases of the eye(s).
During treatment, you may be at risk of developing certain side effects. It is important
you understand these risks and how to monitor for them. See additional information
under Section
6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
DuoTrav should not be used in pregnancy or in women planning to become pregnant.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
It is not recommended to use DuoTrav while breastfeeding.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any
medicines, vitamins or supplements that you buy without a prescription from your pharmacy,
supermarket or health food shop.
Some medicines may interfere with
DuoTrav
and affect how it works.
Tell your doctor if you take any of the following medicines:
medicines used to treat high blood pressure or heart problems, e.g. beta-blockers
(Metoprolol), calcium channel blockers or digoxin, digitalis glycosides
medicines used to treat irregular heartbeats e.g. amiodarone and quinidine
fluoxetine or paroxetine, monoamine oxidase inhibitors (MAOIs) medicines used to treat
depression
narcotics e.g. morphine which is used to treat moderate to severe pain
medicines used to treat severe allergic reaction e.g. adrenaline
medicines used to treat high blood sugar
any other eye drops that are similar DuoTrav or other eye drops used in treatment
of glaucoma.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins
or supplements you are taking and if these affect
DuoTrav.
4. How do I use DuoTrav?
How much to use
Follow the instructions provided and use DuoTrav until your doctor tells you to stop.
Put one drop of DuoTrav once daily at about the same time each day in the affected
eye(s).
Do not put DuoTrav in more than once daily.
If you are using any other eye drops, use the eye drops at least 5 minutes apart.
Tell your doctor or pharmacist if you do not understand your dose.
When to use DuoTrav
DuoTrav should be used at about the same time each day unless your doctor tells you
otherwise.
How to use DuoTrav
Sitting or lying down might make administration of your eye drops process simpler.
Remove contact lenses if you are wearing them before instilling the eye drops.
Shake the bottle well prior to use.
Follow the steps below to use DuoTrav:
Wash your hands thoroughly with soap and water.
Before using a bottle for the first time, tear off the overwrap pouch and take the
bottle out (refer diagram 1).
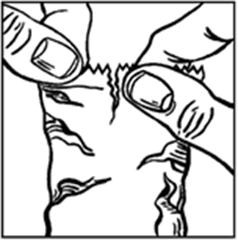
Diagram 1
Remove the cap from the bottle.
Mix the contents of the bottle by inverting 5 to 10 times.
Hold the bottle upside down in one hand between your thumb and middle finger (refer
diagram 2).
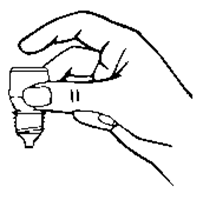
Diagram 2
Tilt your head back, gently pull down the lower eyelid of your eye to form a pouch/pocket.
Place the tip of the bottle close to your eye. Do not touch the tip to your eye as
this may cause injury to the eye.
Do not touch the dropper tip to eyelid or surrounding areas or any surface to avoid
contamination of the dropper tip and solution.
Release one drop into the pouch/pocket formed between your eye and eyelid by gently
tapping or pressing the base of the bottle with your forefinger (refer diagrams 3
and 4).
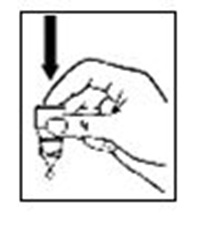
Diagram 3

Diagram 4
Gently close your eye. Do not blink or rub your eye.
When your eye is closed, place your index finger against the inside corner of your
eye and press against your nose for about two minutes. This will help to prevent the
medicine from draining through the tear duct to the nose and throat, from where it
can be absorbed into other parts of your body and may result in less side effects.
This will also help to prevent the unpleasant taste sensation that some people experience
while using these eye drops.
If necessary, repeat the above steps for the other eye.
You may feel a slight burning sensation in the eye shortly after using DuoTrav. If
it continues, or is uncomfortable, contact your doctor or pharmacist.
If you want to use any other eye drops wait at least 5 minutes after putting DuoTrav
in.
It is normal for a small amount of eye drops to spill onto your cheek since your eyelids
can only hold less than one drop at a time. Wipe away any spillage with a tissue.
Replace the cap on the bottle and close it tightly.
Always keep the bottle tightly closed when not in use.
Wash your hands again with soap and water to remove any residue.
Discard DuoTrav 4 weeks after opening it.
If you forget to use DuoTrav
DuoTrav should be used regularly at the same time each day. If you miss your dose
at the usual time, put the drops in as soon as you remember and then go back to using
them as told by your doctor.
If it is almost time for your next dose, skip the dose you missed and take your next
dose when you are meant to.
Do not take a double dose to make up for the dose you missed.
If you use too much DuoTrav
If you think that you have used too much DuoTrav, you may need urgent medical attention.
You should immediately:
phone the Poisons Information Centre
(by calling
13 11 26), or
contact your doctor, or
go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
If you accidentally put several DuoTrav Eye Drops in your eyes, immediately wash your
eyes with warm tap water.
5. What should I know while using DuoTrav?
Things you should do
Tell your doctor immediately if you are going to have any surgery.
Remove soft contact lenses before putting in DuoTrav. Put your lenses back in 15 minutes
after putting in the eye drops.
Call your doctor straight away if you:
notice any change in your eye colour.
have any heart or breathing problems.
have any changes in your eyelashes such as increased length, thickness, pigmentation,
and/or number of lashes or unwanted hollows of the upper eyelid, eyelid skin darkening
after starting the treatment with DuoTrav.
develop any signs of allergic reaction.
become pregnant, are planning to become pregnant or if you are breast feeding while
using DuoTrav.
Remind any doctor, dentist, pharmacist or specialist you visit that you are using
DuoTrav.
Things you should not do
Do not stop using this medicine suddenly.
Do not use DuoTrav in children.
Do not give DuoTrav to anyone else, even if they seem to have the same condition as
you.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how DuoTrav
affects you.
DuoTrav may cause temporary blurred vision after putting in the eye drops in some
people.
If so, then wait until your vision clears before driving or using machinery.
Looking after your medicine
Store below 25 °C
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do
not store it:
in the bathroom or near a sink, or
in the car or on window sills.
Keep it where young children cannot reach it.
When to discard your medicine
Discard DuoTrav 4 weeks after opening it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy
for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of
them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you
have any further questions about side effects.
Less serious side effects
|
Less serious side effects
|
What to do
|
|
Eye problems:
Redness of the eye (s)
Eye pain
Uncomfortable feeling in the eye(s)
Dry eye
Blurred vision
Conjunctivitis
Eye swelling
Excessive secretion of tears from the eye(s)
Eyelid swelling
Eye irritation
Other problems:
Dizziness
Severe headache, dizziness, vision problems
Headache
Cough
Tiredness
Unusual hair loss or thinning
Diarrhoea
Feeling sick
Unusual weakness
Stuffy nose
Skin discolouration
Rash
Dizziness, lightheadedness
Headache, feeling sick, weakness, dizziness may indicate low blood sugar level.
|
Speak to your doctor if you have any of these less serious side effects and they worry
you.
|
Serious side effects
|
Serious side effects
|
What to do
|
|
Eye problems:
Symptoms of allergic reaction may include shortness of breath, difficulty breathing,
swelling of the face, lips, tongue or other parts of the body, rash, hives
Swelling of eye with symptoms such as eye pain, decreased vision, blurred vision
Inflammation or infection of the thin membrane that covers your eye and the inside
of your eyelid.
An eye problem that causes blue, brown or grey discoloration around the iris or on
the white part of the eye
Double vision
Inflammation of the cornea, the clear dome-shaped tissue on the front of the eye,
symptoms include eye pain, redness, eye sensitivity
Droopy eyelid that occurs when the upper eyelid droops over the eye, which can impact
your vision.
Buildup of fluid in the macula, an area at the back of the eye, with symptoms like
blurred vision, distorted vision.
Unwanted hollows of the upper eyelid
Problems with eyelashes such as eyelash discolouration, increased length, thickness,
pigmentation, and/or number of lashes
Other problems:
fast or irregular heart beat
|
Call your doctor straight away, or go straight to the Emergency Department at your
nearest hospital if you notice any of these serious side effects.
|
Tell your doctor or pharmacist if you notice anything else that may be making you
feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can
report side effects to the Therapeutic Goods Administration online at
www.tga.gov.au/reporting-problems . By reporting side effects, you can help provide more information on the safety of
this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop
taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What DuoTrav contains
|
Active ingredient
(main ingredient)
|
40 micrograms (0.004%) travoprost and 5 mg (0.5%) timolol (as timolol maleate 6.83
mg)
|
|
Other ingredients
(inactive ingredients)
|
Ethoxylated hydrogenated castor oil, propylene glycol, boric acid, mannitol, sodium
chloride, sodium hydroxide and/or hydrochloric acid and purified water. The solution
is preserved with polyquaternium-1.
|
|
Potential allergens
|
NA
|
Do not take this medicine if you are allergic to any of these ingredients.
What DuoTrav looks like
DuoTrav is a colourless to light yellow aqueous solution. It comes in 2.5 mL bottle
with or without a pouch.
Australian Registration Number
AUST R: 177772
Who distributes DuoTrav
This product is supplied in Australia by:
Novartis Pharmaceuticals Australia Pty Limited
ABN 18 004 244 160
54 Waterloo Road
Macquarie Park NSW 2113
Telephone 1 800 671 203
® = Registered Trademark
This product is supplied in New Zealand by:
Novartis New Zealand Limited
PO Box 99102
Newmarket
Auckland 1149
New Zealand
Free Phone: 0800 354 335.
This leaflet was prepared in June 2025.
Internal document code:
dut031123c_V2 based on PI dut031123i